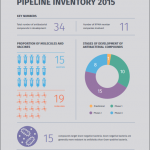
6:00 - 6:30 pm
Registration
6:30 - 6:45 pm
Introductory remarks by Mr. Eduardo Pisani, Director General, IFPMA
6:45 - 8:00 pm
Panel discussion moderated by Ms. Adva Saldinger, Associate Editor, Devex
8:00 - 8:15 pm
Concluding remarks by Dr. Keiji Fukuda, Special Representative for Antimicrobial Resistance, Assistant Director-General, World Health Organization (WHO)

Ms. Adva Saldinger
Associate Editor, Devex
As a Devex Impact associate editor, Adva leads coverage of the intersection of business and international development. From partnerships to trade and social entrepreneurship to impact investing, she enjoys exploring the role the private sector and private capital play in development. Previously, she has worked as a reporter at newspapers in both the U.S. and South Africa. Most recently, she has been ghostwriting a memoir for a former child slave and NGO founder in Ghana.

Mr. Eduardo Pisani
Director General, IFPMA
Eduardo Pisani became Director General of the IFPMA at the end of 2009. He came to the IFPMA from Bristol-Myers Squibb, a company he joined in 2001, and in which he had risen to the position of Vice-President, International Policy and Government Affairs. Prior to that, Mr. Pisani held positions as legal counsel at Immuno AG in Belgium and Austria, at Baxter Healthcare in Belgium and Italy, and in European Policy and Government Affairs at Adamson Associates and SmithKline Beecham in Belgium. He started his career in 1989, in the marketing department of Lederle France. Mr. Pisani graduated in Law at the University of Catania, Italy. As IFPMA Director General, Eduardo leads the dialogue between research-based pharmaceutical companies and associations with the United Nations and its specialized agencies. Together with his team and working with IFPMA President and Vice Presidents, Eduardo shares the expertise and perspectives of the industry in global health discussions. He believes that platforms for constructive policy discussion are essential to effective policy making and to public health, and has been instrumental in shaping many of IFPMA’s leading initiatives such as those on NCDs and on NTDs.

Dr. Keiji Fukuda
Special Representative for AMR, Assistant Director-General, World Health Organization (WHO)
Dr Keiji Fukuda is Special Representative for Antimicrobial Resistance in the office of the WHO Director-General.
From September 2010 until November 2015, Keiji Fukuda served as the Assistant Director-General for Health Security.
He was Special Adviser on Pandemic Influenza to the Director-General from October 2009 to August 2010. Prior to that, he was the Assistant Director-General for Health Security and Environment ad interim. In 2005, he came to WHO as a Scientist in the Global Influenza Programme, then was Coordinator from 2006 to 2008 and then was appointed its Director. Under his guidance, the Programme greatly expanded its global role in providing technical leadership and guidance related to seasonal, avian and pandemic influenza.
Dr Fukuda has extensive public health and research experience working on influenza. At WHO, he has helped shape the global approach to pandemic preparedness, helped manage the response to the influenza H1N1 pandemic, strengthened surveillance, and played an instrumental role in facilitating intergovernmental discussions over the sharing of influenza viruses and related benefits. He has participated in many field investigations including the earliest outbreaks of avian H5N1 influenza and the emergence of SARS.
Before coming to WHO, Dr Fukuda was Chief of the Epidemiology Unit, Influenza Branch at the Centers for Disease Control and Prevention in the United States. He is a physician and received his BA from Oberlin College, MD from the University of Vermont and MPH from the University of California, Berkeley.

Dr. Joe Larsen
U.S. Department of Health and Human Services (HHS), Biomedical Advanced Research and Development Authority (BARDA)
Dr. Larsen is acting Deputy Director of the Biomedical Advanced Research Development Authority (BARDA). He previously served as Deputy Director of BARDA’s Chemical, Biological, Radiological, and Nuclear Medical Countermeasure Division where he oversaw a $2.8B fund for the procurement of medical products for use during public health emergencies. He is also the BARDA lead for the Obama Administration’s Initiative on combating antibiotic resistant bacteria and has chaired intergovernmental working groups on research and development and economic incentives for antibacterial drug development. Previously Dr. Larsen served as Chief of the Broad Spectrum Antimicrobials program at BARDA. The goal of BARDA’s Broad Spectrum Antimicrobials program is to develop additional antimicrobial treatment options needed to counter the growing threat of antimicrobial resistance. In that role, he oversaw a portfolio of approximately $1.2B in programs that support the development of novel antibacterial and antiviral drugs. Dr. Larsen also serves as the BARDA representative on the U.S. Transatlantic Task Force on Antimicrobial Resistance. Previously, Dr. Larsen served as a Senior Science and Technology Manager at the Joint Science and Technology Office for Chemical and Biological Defense (JSTO-CBDP) within the Defense Threat Reduction Agency (DTRA). There, he managed a ~$50M applied research program aimed at the development of medical therapeutics against viral, bacterial, and toxin threat agents. From 2005-2006, Dr. Larsen was an American Association for the Advancement of Science (AAAS) fellow at the Department of Homeland Security. There, he managed University based research programs aimed at the development enhanced food safety detection systems and medical countermeasures for agricultural threat agents. Joe was a 2005 National Academy of Science Christine Mirzayan fellow with the Board of Life Sciences. Dr. Larsen received his PhD in Microbiology from the Uniformed Services University of the Health Sciences and his BA with honors from the University of Kansas.

Dr. Manica Balasegaram
Global Antibiotic Research and Development (GARD) Partnership
Dr Manica Balasegaram, who is a medical doctor by training, started his career in internal and emergency medicine in the UK and in Australia, then joining Médecins sans Frontières as a field physician in Sub-Saharan Africa and Southern Asia. He was later appointed Head of the MSF Manson Unit, before initially joining DNDi as Head of Leishmaniasis – a position he left four years ago to become the MSF Access Campaign’s Executive Director. His experience spans clinical and public health practice in neglected and infectious diseases, clinical trials and drug development, international work on health policy and access to medicines and involvement in a variety of expert technical committees. He rejoined DNDi in June 2016 as Director of the Global Antibiotic Research and Development Partnership, a joint DNDi-WHO initiative.

Ms. Takuko Yamada-Sawada
Director of the Board, Senior Executive Officer, Senior Vide President, Corporate Strategy Division, Shionogi & Co., Ltd.
Ms. Takuko Yamada-Sawada has been Senior Managing Executive Officer and General Manager of Management Strategy Division at Shionogi & Co., Ltd. since April 2011 and June 2015 respectively. Ms. Yamada-Sawada served as Senior Executive Officer of Shionogi & Co. Ltd. since April 1, 2013 and served as its Senior Vice President of Global Development for Pharma. She has had a long interest in bringing innovative medicines to market, improving R&D efficiency and globalization. She served as an Executive General Manager of Global Development Office - Pharmaceutical Development Division at Shionogi & Co., Ltd., since April 1, 2013. She served as an Executive Officer of Shionogi & Co. Ltd., and also served as its Executive General Manager of Pharmaceutical Development Division until April 1, 2013. Ms. Yamada-Sawada has been a Director of TransCelerate BioPharma Inc., since June 1, 2014. She serves as Director of Shionogi & Co. Ltd.

Mr. Yasuhide Yamada
Councillor, Director-General, Office for Pandemic Influenza and New Infectious Diseases Preparedness and Response, Coordination Office of Measures on Emerging Infectious Diseases (EIDs), Cabinet Secretariat, Government of Japan (Video Link)
Yasuhide Yamada’s academic background is environmental science and policy administration. He has a Master of Science from Hokkaido University (Japan) and a Master of Public Management from Carnegie Mellon University (USA).
Yamada, as an officer of Ministry of Economy, Trade, and Industry (METI), has long and diversified career in policy-making of science and technology and innovation (including medical and pharmaceutical fields), electronics/IT/cyber security, energy and environment (global warming issue) etc.. He also experienced negotiation of Economic Partnership Agreement, and nuclear power litigation.
Since July 2014, Yamada leads the basic policy of Pandemic Influenza and Emerging Infectious Diseases (EIDs) including Ebola, MERS, Zika, and AMR from the perspective of total coordination and national security.

Dr. Adrian Thomas
Global Head Health Economics & Market Access Medical Devices&Vice President, Global Public Health Johnson & Johnson
Adrian Thomas is the Global Head of Health Economics & Market Access for the Medial Device companies of Johnson & Johnson, and also serves as Vice President, Global Programs Strategy & Evaluation within the Johnson & Johnson & Johnson Global Health organization.
Adrian has a special interest in the fields of public health, commercial and market access strategy, and pharmaceutical policy. He has also held numerous roles in the market access including Global Head of Market Access for Janssen, the pharmaceutical companies of Johnson & Johnson, as well as roles in risk management and drug safety, including Global Head of Benefit Risk Management and Chief Safety Officer for Janssen.
Prior to joining J&J, Adrian held roles in regional medical affairs, drug development and product management for Schering-Plough and Eli Lilly. He is a clinical pharmacologist and vascular physician with experience in clinical trials design and methodology.
Adrian is a Fellow of the Royal Australasian College of Physicians and the College of Medial Administrator’s. He received his Bachelor of Medicine and Bachelor of Surgery degrees from the University of Melbourne in Australia.
He is based in New Hope, PA.
21st Sep 2016
18:30 to 20:15 - Reception to follow UTC−05:00
Convene, New York

News Releases
NEW YORK, 20 September 2016 (CSRWire) – In the side-lines of the United Nations General Assembly High-level Meeting on Antimicrobial ...
Read More
News Releases
Leading Pharmaceutical Companies Present Industry Roadmap to Combat Antimicrobial Resistance Comprehensive roadmap lays out key commitments the pharmaceutical companies pledge ...
Read More