Strengthening
Regulatory Systems
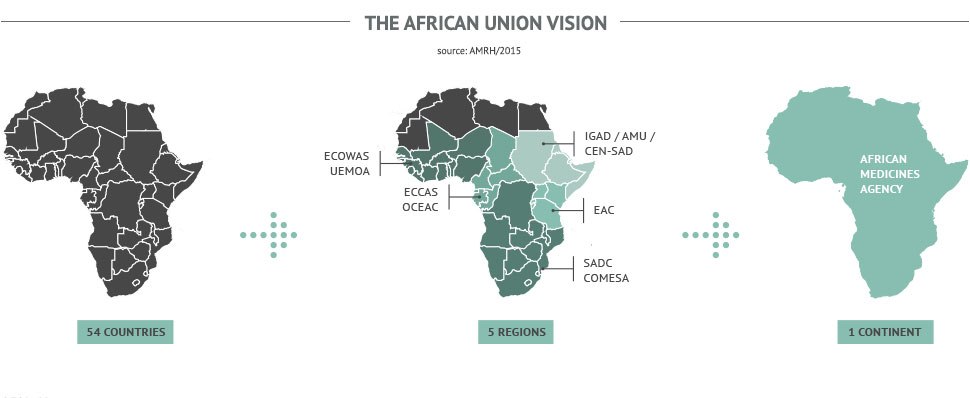
The Africa Regulatory Network (ARN)





The Africa Regulatory Network (ARN)
The ARN is an ad-hoc network of our organization. The Network works in partnership with regulatory authorities, the pharmaceutical industry in Africa, and relevant regulatory stakeholders to encourage greater harmonization and convergence of regulatory requirements. The ARN’s goal is to help enable faster and expanded availability of quality, innovative medical products for patients.

![]()
Building Africa Capacity
![]()
For In Sync Regulatory Systems
![]()
Through the 6 C's
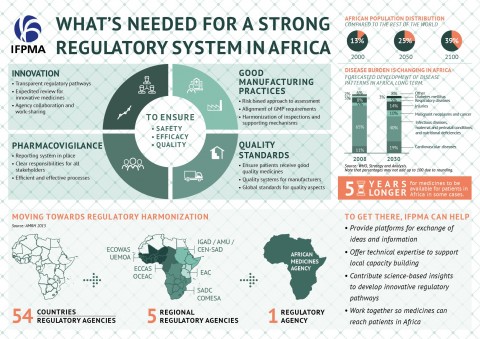
We believe that people in Africa should have timely access to quality medicines and vaccines that have proven efficacy and safety. To ensure this, consistency and predictability of regulatory systems are needed. These systems are composed of standards, regulations, guidelines, specifications and procedures aimed at keeping access to safe medicines and vaccines on track. Weak or absent regulatory systems can lead to low quality or fake medical products; inadequate monitoring of side-effects; and often delay in the availability of vital medical products to patients.
The Africa Regulatory Network (ARN)

Pillar 1: What we do: Building Capacity in Africa
Help build science-based regulatory insights
- To illustrate how African regulatory stakeholders can base regulatory pathways on sound science, advancements in technology and innovation,
- To pave the way towards harmonization and convergence, and
- To provide technical assistance for countries to tackle priority regulatory issues.
Help address gaps
- To provide the necessary support to national and regional medical product regulations in:
- norms and standards setting,
- capacity building,
- regulatory pathways development and assessment (e.g. pre-qualification),
- product development review (e.g. clinical trials, manufacturing, and control),
- post-marketing surveillance (pharmacovigilance, quality issues such as decreased efficacy),
- building platforms for networking and information exchange.
- To facilitate ongoing development and resource sharing on the above to enable stakeholders across the spectrum to contribute their own capacity, strength, and expertise; and
- To take part in a meaningful dialogue and call for greater consideration of regulatory issues in health policies development in Africa.
Without effectively addressing these gaps, the full potential of the many biomedical initiatives in Africa will not be achieved in the years ahead.
Driver for change in the regulatory landscape
- To challenge old paradigms and promote innovation to both medical products development and regulations. This includes developing sound regulatory approaches to manage benefit-risk, dynamically monitor as well as prioritize scientific advances and their impact, and
- To demonstrate how regulatory stakeholders can build on their expertise and strength towards harmonization, convergence, coordination, and communication to address capacity issues in a complementary and pragmatic manner.
Operating with pragmatism and a reality that fits Africa
- To gain a better understanding of issues different players have to deal with, such as ongoing public health challenges, ethical issues, commercial realities as well as public and political priorities, and
- To translate this understanding into practical approaches that address significant regulatory issues and positively impacts patients.
The Africa Regulatory Network (ARN)
Pillar 2: Why We Do it: For in sync regulatory systems
Regulatory systems should be in sync: Why? Simply because:
- In an increasingly globalized world, no single regulatory stakeholder can meet current regulatory challenges alone. Africa is not exempted from these global challenges.
There are different opportunities for convergence and the underlying commitment on the need to work together to address common public health issues has become much clearer and stronger.
ARN aims at:
- Placing the World Health Organization resolution on Regulatory System Strengthening at the heart of regulatory systems improvement, and as a key driver to bring regulatory innovation in Africa;
- Building the case that the pharmaceutical industry, through ARN is a key player in regulatory system strengthening;
- Learning from and showcasing success stories of harmonization efforts undertaken worldwide, and specifically in Africa to feed on potential new initiatives;
- Helping build strong evidence on how commitment to regulatory excellence is key to addressing gaps in expertise;
- Raising awareness on how the regulatory challenges industry faces across the registration process, the manufacturing chain, and throughout products’ life-cycle management can hamper availability of good quality medicines and vaccines; and
- Demonstrating how global initiatives and systems innovation all aim at promoting convergence of global standards; reducing disparities in registration; assessing and enhancing new technology; fulfilling unmet medical needs; and facilitating greater transparency in tandem with personal data protection.
The Africa Regulatory Network (ARN)
Pillar 3: How we do it: Through the 6Cs
The concept of the 6C’s hails from longstanding regulatory convergence experience in Asia. Its principles place Cooperation, Collegiality, Capacity, Commitment, Communication and Convergence at the heart of effective and sound harmonization/convergence approaches. This concept has full relevance to any other region of the world embarking on same efforts, including Africa.
We call for the 6 Cs to drive the regulatory agenda. Here is why.
Cooperation
- helps address regulatory agencies’ resources constraints, and
- Avoids duplication of work that compromises efficiency and facilitates expertise-sharing.
Collegiality
- Finds common goals and solutions to collectively address public health needs, and
- Creates platforms to facilitate trust building.
Capacity
- Addresses gaps in regulatory capabilities and development needs such as:
- Technical and management knowledge; and
- Development of learning and exchange-information platforms.
- Encourages innovation in regulatory science to find pragmatic solutions.
Commitment
- Enables an adequate allocation of resources to regulatory functions, and
- Supports active and credible engagement in collaborative initiatives.
Communication
- Increases transparency and predictability throughout the regulatory process, and
- Facilitates the exchange of information.
Convergence
- Facilitates regulatory requirements coherence,
- Limits country specific issues, and
- Enables global acceptance and oversight.