- Breakthroughs in the treatment of hepatitis C pose questions on how to make innovations more available to a large number of patients. A new report looks at possible innovative funding solutions for low and middle-income countries (LMICs) to scale up treatments for viral hepatitis and support health systems to deliver them.
- The report reviews over 20 different existing funding mechanisms, but finds no one single innovative mechanism is best. Instead, a mixture of approaches that consider the national context offer considerably more promise.
- The report states that successful innovative finance mechanisms require collaboration between payers, patients and healthcare providers. Political commitment to control viral hepatitis through a comprehensive public health approach is critical to ensure interest from prospective funders.
16th April 2016, Barcelona│To coincide with the 2016 International Liver Congress in Barcelona, the Viral Hepatitis Prevention Board (VHPB) launches a report that reviews over 20 different existing funding mechanisms and examines how these financing sources could be used to support government strategies to provide access to treatment to patients suffering from viral hepatitis in low-and middle-income countries (LMICs). The purpose of the report is to increase understanding of the challenges and opportunities for LMICs of working with stakeholders in this new area of financing. The report was commissioned by the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA).
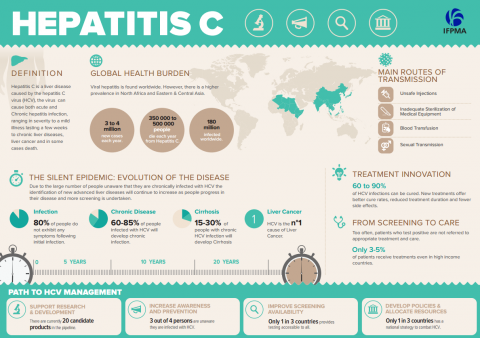
Recent scientific breakthroughs in the treatment for hepatitis C, and the broader objectives of the health-related Sustainable Development Goals (SDGs) call for new approaches and new players to help find innovative funding mechanisms to scale up treatments for hepatitis and support health systems in delivering them in LMICs. Worldwide, over 400 million people live with chronic hepatitis B or C and LMICs are particularly affected with many countries experiencing high levels of infection, precarious prevention of transmission and low access to treatment.
The report surveyed over 250 organizations that deal with (innovative) financing and/or health care. It identifies a number of financing mechanisms that offer considerably promise, including non-infrastructure public private partnerships that build on social impact bond funding, shared value projects, and micro-financing. However, focusing on only one single financing mechanism such as a global “hepatitis fund” meeting all the challenges for treatment and prevention of hepatitis in LMICs is unlikely to be feasible.
Optimal conditions for setting up new financing mechanisms will require political support for a viral hepatitis national plan, and competence in selecting the mechanisms best adapted to country-specific challenges and that meet the needs of all the stages of the therapy cycle. To pursue the opportunities identified in the report, VHPB recommends convening a multi-stakeholder group involving regional banks, regional leadership both from a public health and a financing perspective, investors, patients, health professionals and services, pharmaceutical and diagnostics industries and independent assessors and evaluation advisors, each with potentially unique roles to play.
“At national level, support is crucial to find funding solutions and regional groups are uniquely positioned from a political and a financing perspective to provide leadership. Let us not lose sight of our goal: to provide prevention and access to treatment to patients suffering from hepatitis”, said Professor Pierre van Damme, VHPB Executive Secretary and professor at the University of Antwerp, Faculty of Medicine and Health Sciences.
“Our experience in LMICs has shown how important it is to espouse approaches that are not top down, but that empower actors at regional level to better promote government ownership. These successful approaches are sustainable, and contribute to increasing local capacity through partnerships”, said Eduardo Pisani, Director General of the IFPMA.
To full report can be access here.
For further information contact:
Pierre Van Damme │ Executive Secretary VHPB │Pierre.vandamme@uantwerpen.be │+32 3 265 47 11 or +32 476 20 07 54│ Peter De Meyer │ communication department │ University of Antwerp│
Mario Ottiglio │ Director, Public Affairs & Global Health Policy, IFPMA │M.Ottiglio@ifpma.org │+41 22 338 32 00
About VHPB:
The Viral Hepatitis Prevention Board (VHPB) is an international board of experts in viral hepatitis, offering a platform for dissemination of scientific information related to viral hepatitis. Board members meet twice a year to discuss technical and country specific issues. The Board has a permanent scientific secretariat, located at the Centre for the Evaluation of Vaccination (CEV) of the University of Antwerp.
About IFPMA:
IFPMA represents the research-based pharmaceutical companies and associations across the globe. The research-based pharmaceutical industry’s 1.3 million employees research, develop and provide medicines and vaccines that improve the life of patients worldwide. Based in Geneva, IFPMA has official relations with the United Nations and contributes industry expertise to help the global health community find solutions that improve global health.